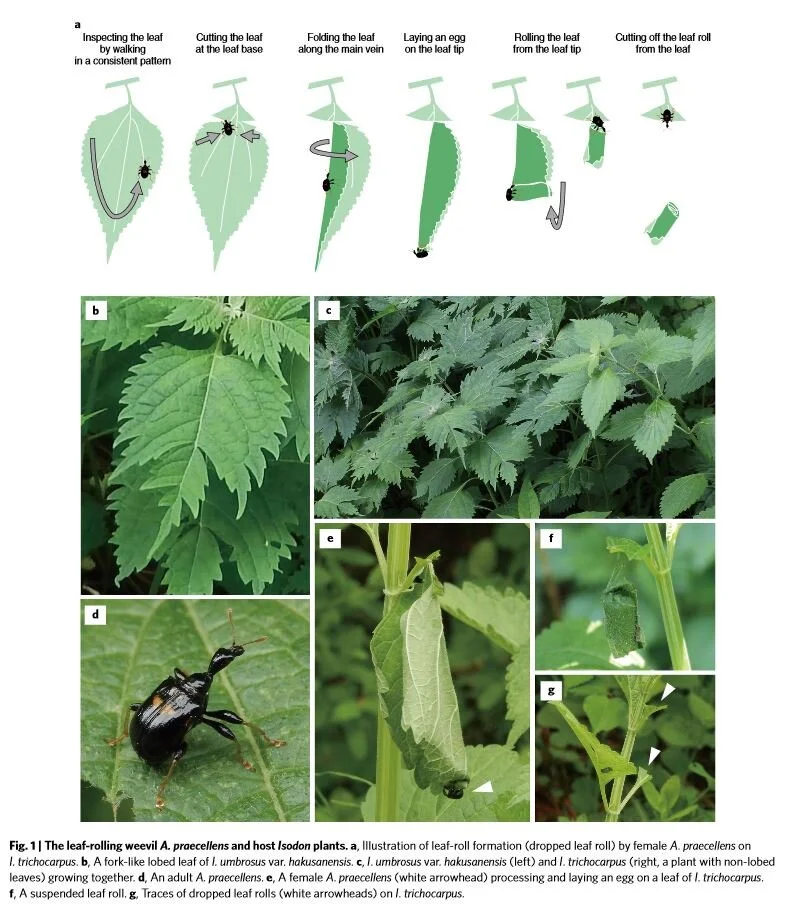
Plants can defend themselves from herbivores in a variety of ways - thorns, spines, hairs, toxins, etc. - but have you ever considered the role of leaf shape in preventing herbivory? It’s okay if you haven’t because leaf shape rarely, if ever, makes it into conversations of plants defense. A recent experiment from Japan has changed that by demonstrating that leaf shape can actually deter a specialist leaf-rolling weevil.
Meet Apoderus praecellens, a leaf rolling weevil that specializes on a genus of mints called Isodon. To successfully reproduce, female leaf rolling weevils must roll up an Isodon leaf while laying eggs as she goes. The end result is a tiny cigar-shaped, edible nursery chamber in which her larvae will develop. The act of processing a leaf is a complex process.
Isodon trichocarpus Photo by Qwert1234 licensed by CC BY-SA 3.0
The female weevil begins by walking along the margin of the leaf until she reaches the apex. At that point she walks sideways towards the interior of the leaf until she finds the midrib. She then turns around and walks back toward the leaf base again. She repeats these steps several times on both sides of the leaf until she is satisfied. At that point, she will take several bites out of the midrib, which causes the leaf to wilt. The wilted leaf is then much easier to manipulate and thus the rolling process begins.
In the wild, female weevils are well documented on the leaves of I. trichocarpus but not on the leaves of I. umbrosus. This is strange because not only are these plants closely related, they frequently grow in close proximity to one another. Why would the female weevils prefer one over the other? The answer appears to lie in the shape of their leaves.
Isodon trichocarpus produces non-lobed leaves whereas the leaves of I. umbrosus are deeply lobed. When presented with a choice, female weevils did indeed choose to roll I. trichocarpus leaves over those of I. umbrosus. These plants do not differ in their chemical makeup and larvae raised on both species did not differ in their health or development time. Thus, nutritional value or defense compounds don’t explain weevil preference.
Even more amazing is that the preferences seemed to change when I. trichocarpus leaves were cut to resemble the lobed I. umbrosus leaves. It seems that the presence of leaf lobes is the key to whether a weevil decides to lay her eggs or not. The reason for this seems to be the complex leaf inspection behavior outlined above. The deep lobes of I. umbrosus leaves disrupt the female weevils as they carry out their complex inspection process. If the females are interrupted, they rarely progress to the leaf rolling stage.
The researchers are quick to point out that leaf shape in this instance probably didn’t evolve in response to herbivory. Leaf shape is the result of a multitude of selection pressures like light availability, heat, and drought. Still, the fact that leaf shape can also influence herbivore pressure is an interesting piece to add to the puzzle. It is a great reminder that an organism’s niche comprises so much more than simply the abiotic conditions in which it lives. The niche is also the myriad biological interactions each organism undertakes.
Further Reading: [1]