It is a rare but special day when we can add a new plant to the relatively small list of carnivorous plants. It is even more exciting when that plant has been “hiding” in plain sight all this time. Meet the western false asphodel (Triantha occidentalis), a lovely monocot native to nutrient-poor wetlands in western North America.
Triantha occidentalis may seem like an odd carnivorous plant. At first glance, it doesn’t have much in the way of carnivorous adaptations; there are not pitfall traps, no sticky leaves, no snap traps, and no bladders anywhere on the plant. However, if you were to examine this species during its flowering season, you would notice that a lot of small insects seem to get stuck to its flowering stem.
Indeed, the ability of this species to trap insects has been known for quite some time. Even old herbarium collections of T. occidentalis are chock full of insect remains stuck to the scape. Magnify the flowering stem and you will see that it is covered in sticky hairs or trichomes that look a lot like miniature versions of those covering the leaves of more obvious carnivores like sundews (Drosera spp.). Observations such as these led scientists to investigate whether this wonderful little wetland monocot actually benefits from trapping all those arthropods.
Via a series of experiments using isotopes of nitrogen, scientists have revealed that T. occidentalis really does obtain a nutritional boost from the insects it traps. This isn’t a passive process on the part of the plant either. It was also discovered that the plant also secrets the digestive enzyme phosphatase, which helps break down the trapped insects. When the team examined what was going on within the tissues of the plant, they found even more evidence of its carnivorous nature.
Look closely and you can see sticky glands and trapped insects just below the flowers! Photo by Michael Kauffmann (www.backcountrypress.com)
It turns out that 64% of the nitrogen within the plant is obtained via insect digestion, which is comparable to that of other known carnivorous plants such as the aforementioned sundews. Interestingly, it appears that the insect nitrogen the plant obtains is first stored in the flowering stem and fruits but is then transported down into the roots and rhizome underground to be utilized in the following growing season. Why exactly the plant does this requires further investigations. Perhaps by using its flowering stems to obtain nutrients that are in short supply in its wetland habitat, the plant is able to better offset the cost of flowering each year.
By far the most remarkable aspect of this discovery is where carnivory occurs on the plant. With few exceptions, the vast majority of carnivorous plants keep their feeding organs away from their flowers. The leading hypothesis on this suggests that separating feeding and reproduction in space (and sometimes time) helps carnivorous plants avoid catching and digesting their pollinators. However, T. occidentalis does the opposite. It produces all of its sticky hairs very close to its blooming flowers.
Large floral visitors like butterflies appear to be the main pollinators and are too large to get stuck, whereas smaller insects like midges do. Photo by Michael Kauffmann (www.backcountrypress.com)
The key to this apparent morphological contradiction may lie in the stickiness of those hairs. It has been observed that the vast majority of insects trapped on the flowering stems of T. occidentalis are mostly midges and other small insects that don’t function as pollinators for the plant. It is possible that the larger bees and butterflies that could function as true pollinators are simply too large and strong to be trapped. Again, more research is needed to say for sure.
All in all, T. occidentalis represents a unique carnivorous plant whose true nature required solid natural history knowledge and observation to reveal. The fact that we are just learning about its carnivorous habit after all this time suggests that many more potentially carnivorous plants may also be “hiding” in plain sight (I’m looking at you, Silene), waiting for curious minds to collect the necessary data. This is also an exciting discovery from a taxonomic perspective as well. Up until now, all of the known carnivorous monocots hail from the order Poales. Therefore, T. occidentalis represents the first non-Poalean carnivorous monocot! For all these reasons and more, I am excited about future research on this plant and others like it.
Further Reading: [1]








![[SOURCE]](https://images.squarespace-cdn.com/content/v1/544591e6e4b0135285aeb5b6/1615476638537-DVF512X71F5WIY3MR9GT/mcaa204f0001.jpeg)





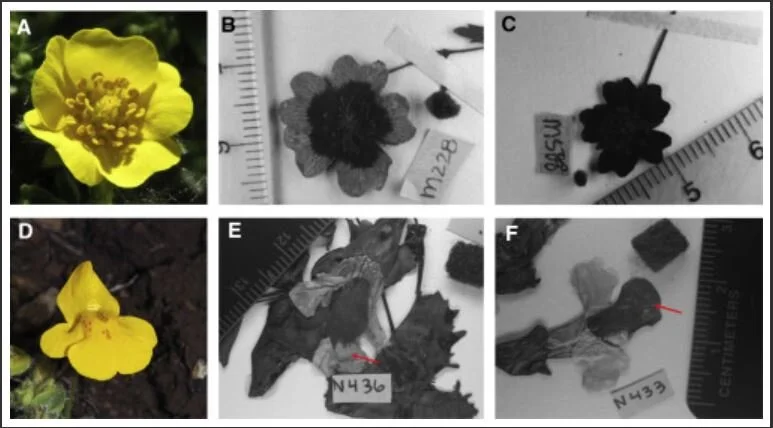
![[Source]](https://images.squarespace-cdn.com/content/v1/544591e6e4b0135285aeb5b6/1614349162208-KK6RR3PXF0XX704I2KX2/1-s2.0-S0960982220316559-fx1.jpg)







![(A–C) Female Forcipomyia biting midge. Arrows indicating pollen grains. [SOURCE]](https://images.squarespace-cdn.com/content/v1/544591e6e4b0135285aeb5b6/1602516996716-ICQ7PIZDYU1BOSTTEWJ3/tacca+midge.JPG)



![The recently described Cremanthodium wumengshanicum growing at elevation in Yunnan, China. [SOURCE]](https://images.squarespace-cdn.com/content/v1/544591e6e4b0135285aeb5b6/1595452902740-1SHVHLYLHXN0VGCR17C4/Cremanthodium_wumengshanicum-novataxa_2015-L._Wang-C._Ren_et_Q.E._Yang.jpg)
![Investigating pollinator visitation among different species of Zaluzianskya. [SOURCE]](https://images.squarespace-cdn.com/content/v1/544591e6e4b0135285aeb5b6/1595453007802-011KTFTH4MG6YEFKCL9M/nph13746-fig-0001-m.jpg)





![Waveforms of male signals for nine species in the Enchenopa binotata complex based on host tree identity [SOURCE].](https://images.squarespace-cdn.com/content/v1/544591e6e4b0135285aeb5b6/1594054293879-I5KJI60173XWLJ6OP7HB/Varying_male_signals_that_species_of_Enchenop_binotata_make.png)























