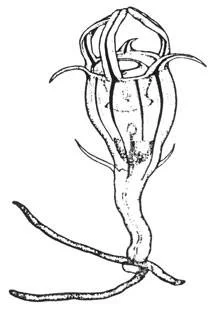
Rare but locally abundant has to be the only proper way of describing the distribution of this peculiar little orchid. I have known about the three birds orchid (Triphora trianthophoros) for some time now. I'm generally not a jealous person but I did find myself quite envious of those who have encountered it. Even with ample herbarium records I simply could not seem to locate any individuals of this species.
The best advice for finding it that I was ever given was to not go looking for it. This secretive little plant is something you almost have to stumble upon. And stumble I did. While surveying some vegetation plots that I had combed over all summer back in 2016 I noticed something new poking up. The slender red stalks had tiny green leaves and elongated flower buds at the top. I knew instantly that this could only mean one thing - I had finally found some three birds.
Both the common and scientific name hint at the fact that these plants are often seen with three flowers. This is not a rule by any means as plants can be found with as few as one flower or as many as 10. Regardless of the amount, finding them is only part of the battle. The other challenge is to catch them in bloom.
The secretive nature of this orchid has led to some interesting tips on how to get your timing right. Some say to check a known population after the first big rain of August. Another more pervasive tip claims that one must take to the forest after nighttime temperatures take a sudden dip. Despite this entertaining advice, it would seem that you just have to be in the right place at the right time.
What is known about the flowering habits of the three birds orchid is that populations tend to flower in unison. The buds all develop to a certain point and stop. They will sit and wait for the right conditions (whatever they might be) to arise. Once that crucial condition is hit, they rapidly bloom en masse. This is a wonderful strategy for a flowering plant that lives tucked away on the shady forest floor.
Concealed among the forest debris, one or two flowers wouldn't get much attention. Hundreds of bright white and pink flowers, however, certainly do! Juxtaposed against the shade of the forest, these little orchids almost glow like little neon signs. Despite this mass effort, it has been found that pollination rates are usually very low. Instead, this orchid most often reproduces vegetatively by budding off tiny plantlets from the main root stock. Because of this, it is not uncommon to find literally hundreds of plants of various sizes clustered together within inches of each other. This is an impressive sight to behold.... again, if you are lucky enough to find it.
Like many of its orchid cousins, this species is no stranger to the disappearing act. Because they rely so heavily on mycorrhizal fungi for their nutrient needs, exhausted plants will often go dormant under the soil for years until they gain enough energy to produce stems, leaves, and flowers again. If you come across the three birds orchid during your travels, do yourself a favor and take some time to relish the moment. It may be a long time before you ever see them again.






















![Stems climbing on fallen dead wood (a) or on standing living trees (b). A thick and densely branched root clump (c) and thin and elongate roots (d) [Source]](https://images.squarespace-cdn.com/content/v1/544591e6e4b0135285aeb5b6/1518471929195-C99KP24U24J238YMX055/myco+roots.JPG)










![[SOURCE]](https://images.squarespace-cdn.com/content/v1/544591e6e4b0135285aeb5b6/1485992214448-W3DN42037E3R1Z3OYFIJ/image-asset.jpeg)


![[SOURCE]](https://images.squarespace-cdn.com/content/v1/544591e6e4b0135285aeb5b6/1481672374887-4CWM24UJ7TODRF9CNMCT/image-asset.gif)