Magnolia denudata. Photo by 阿橋 HQ licensed under CC BY-SA 2.0
There are a lot of reasons to like magnolias and floral thermogenesis is one of them. That’s right, the flowers of a surprising amount of magnolia species produce their own heat! Although much more work is needed to understand the mechanisms involved in heat generation in these trees, research suggests that it all centers on pollination.
Magnolias have a deep evolutionary history, having arose on this planet some 95+ million years ago. Earth was a very different place back then. For one, familiar insect pollinators like bees had not evolved yet. As such, the basic anatomy of magnolia flowers was in place long before bees could work as a selective pressure in pollination. What were abundant back then were beetles and it is thought that throughout their history, beetles have served as the dominant pollinators for most species. Indeed, even today, beetles dominate the magnolia pollination scene.
Magnolia sprengeri. Photo by Aleš Smrdel licensed under CC BY-NC 2.0
Beetles are generally not visiting flowers for nectar. They are instead after the protein-rich pollen within each anther. It seems that when the anthers are mature, beetles are very willing to spend time munching away within each flower, however, keeping their attention during the female phase of the flower is a bit trickier. Because there are no rewards for visiting a magnolia flower during its female phase, evolution has provided some species with an interesting trick. This is where heat comes in.
Though it varies from species to species, thermogenic magnolias produce combinations of scented oils that various beetles species find irresistible. That is, if they can pick up the odor against the backdrop of all the other enticing scents a forest has to offer. By observing floral development in species like Magnolia sprengeri, researchers have found that as the flowers heat up, the scented oils produced by the flower begin to volatilize. In doing so, the scent is dispersed over a much greater area than it would be without heat.
Magnolia tamaulipana. Photo by James Gaither licensed under CC BY-NC-ND 2.0
Unlike some other thermogenic plants, heat production in magnolia flowers doesn’t appear to be constant. Instead, flowers experience periodic bursts of heat that can see them reaching temperatures as high as 5°C warmer than ambient temperatures. These peaks in heat production just to happen to coincide with the receptivity of male and female organs. Also, only half of the process is considered an “honest signal” to beetles. During the male phase, the beetles will find plenty of pollen to eat. However, during the female phase, the scent belies the fact that beetles will find no reward at all. This has led to the conclusion that the non-rewarding female phase of the magnolia flower is essentially mimicking the rewarding male phase in order to ensure some cross pollination without wasting any energy on additional rewards.
The timing of heat production also changes depending on the species of beetle and their feeding habits. For species like the aforementioned M. sprengeri, which is pollinated by beetles that are active during the day, heat and scent production only occur when the sun is up. Alternatively, for species like M. tamaulipana whose beetle pollinators are nocturnal, heat and scent production only occur at night. Researchers also think that seasonal climate plays a role as well, suggesting that heat itself may be its own form of pollinator reward in some species. Many of the thermogenic magnolias bloom in the early spring when temperatures are relatively low. It is likely that, aside from pollen, beetles may also be seeking a warm spot to rest.
Personally, I was surprised to learn just how many different magnolias are capable of producing heat in their flowers. When I first learned of this phenomenon, I thought it was unique to M. sprengeri but I was wrong. We still have a lot to learn about this process but research like this just goes to show you that even familiar genera can hold many surprises for those curious enough to seek them out.







































![[SOURCE]](https://images.squarespace-cdn.com/content/v1/544591e6e4b0135285aeb5b6/1509465408526-PS04Q0I13MTWTXHU96XE/image-asset.jpeg)
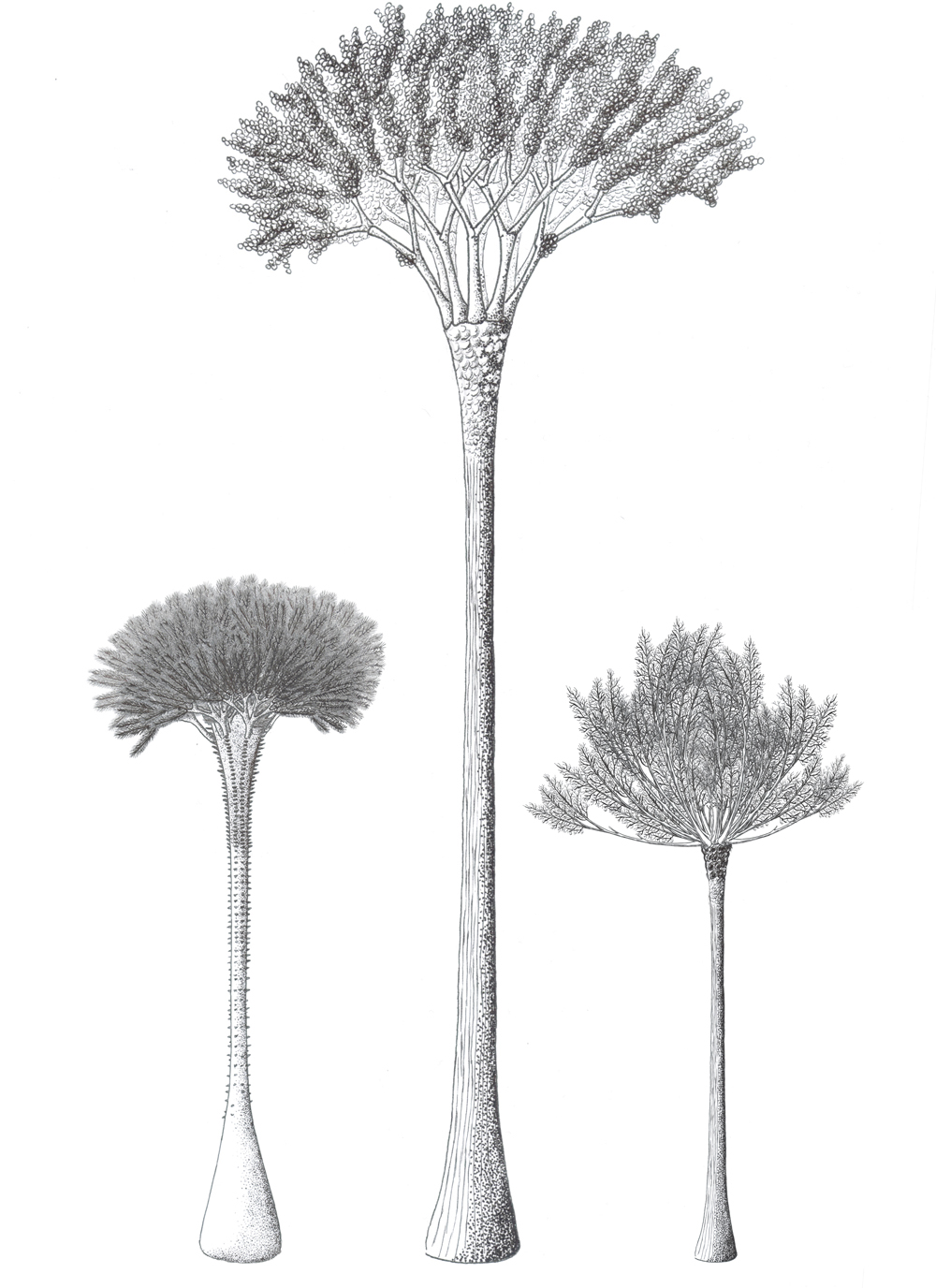
![A cross section of a Cladoxylopsid trunk showing the hollow center, individual xylem strands, and the network of connective tissues. [SOURCE]](https://images.squarespace-cdn.com/content/v1/544591e6e4b0135285aeb5b6/1509465418599-SBMOI12IRYJPVDFDRKZ8/F3.large.jpg)










