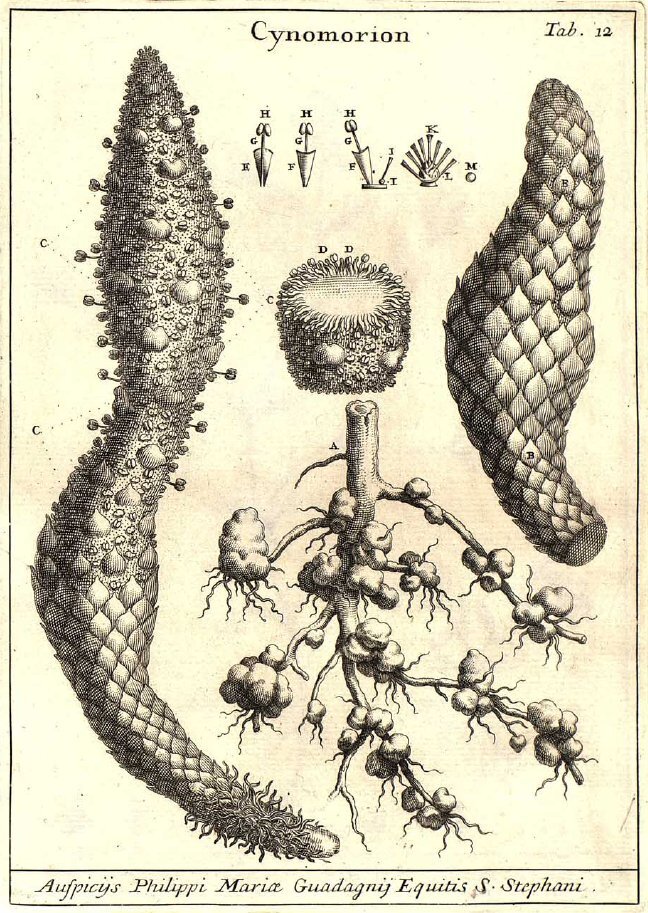
Bearcorn (Conopholis americana) is one of those plants that really challenges mainstream assumptions of what a plant should look like. It produces no leaves, no chlorophyll, and all you ever see of it are its strange reproductive structures. One can easily be forgiven for thinking they had encountered some type of fungus.
Bearcorn is an obligate parasite on oak trees. It simply can’t exist without access to oak roots. From what I have been able to gather, the preferred hosts of bearcorn are the red oaks (section Lobatae). That is not to say the exceptions have not been documented. At least one author claims to have found bearcorn attached to the roots of a white oak (Quercus alba) and even earlier work suggests that American chestnut (Castanea dentata) may have served as an occasional host as well. Regardless, if you want to find bearcorn in the woods, you would do well to search out red oaks first.
According to those who have run germination trials, bearcorn seeds must be in close proximity to oak roots in order to germinate. Some sources say that direct contact is needed whereas others claim that seeds have to be close enough to detect root presence. It is likely that some sort of chemical cue is what initiates the process and this makes sense. For a plant that relies completely on another plant for its water and nutritional needs, it doesn’t make much sense for bearcorn seeds to germinate anywhere but near oak roots.
Upon germinating, the tiny seedling needs to act fast before its meager energy reserves are exhausted. If lucky, the growing seedling will come into contact with an oak root and begin developing a strange organ referred to as the nodule or tubercle. Thus begins its parasitic lifestyle. The tubercle continues to grow throughout the life of the plant, developing into an amorphous, woody blob that continues to envelope more and more oak roots. Its within the tubercle that all of the parasitism takes place.
Cells within the bearcorn tubercle penetrate into the vascular tissues of the oak root, stealing all the water and nutrients the plant will ever need. Over time, the bearcorn tubercle coaxes the roots of the oak to fan outward like the crown of a tiny tree. In doing so, bearcorn is effectively increasing the amount of surface area available to make more parasitic connections. Apparently this all comes at great cost to the oak roots. Over time, oak root size within the tubercle greatly diminishes until some completely perish. Considering the size of some bearcorn populations, one could expect the oak host to fight back.
Indeed, it would appear that oaks are not helpless against bearcorn infestations. Examination of the cells within bearcorn tubercles revealed that as the parasite grows, the oak will begin flooding the infected cells with tannin-rich compounds. Apparently this serves to slow the flow of water and nutrients into the tubercle. There is even evidence that some of those tannins are transferred into the bearcorn tubercle, leading some to suggest that the oak is literally poisoning its bearcorn parasites, albeit slowly.
There is a strong possibility that such oak defenses lend to the relatively short lifespan of bearcorn plants. In at least one study I read, no bearcorn individuals over 13 years of age were found and the average age is estimate to be about 10 years. Perhaps just over a decade is about all a bearcorn can hope for once the its oak host begins to fight back. Good thing bearcorn populations can be surprisingly fecund.
Bearcorn plants reach reproductive maturity at after about 3 years of growth. They flower in the spring and that is when they are at their most obvious. Numerous thick, finger-like stems emerge from the ground covered in whirls of cream-colored, tubular flowers. Though a dense population of flowering bearcorn may look like a bonanza for pollinators, they don’t seem to attract a lot of attention. From what I was able to find, bumblebees are pretty much the only insects to visit the flowers, and even then, visitation rates are low. Apparently bearcorn flowers do not produce any detectable scent nor are they full of nectar. I guess the only real reward is a meager helping of pollen.
Photo by Joshua Mayer licensed under CC BY-SA 2.0
No matter, bearcorn has a nice reproductive trick to ensure plenty of seeds are produced each year - it selfs. The anatomy of the flowers is such that, at maturity, the anthers are in direct contact with the stigma. Even if nothing visits a bloom, it will still go on to clone itself year after year. Once fertilized, each flower gives way to a large fruit chock full of seed. This is where the corn part of the name bearcorn comes from. A stem thick with fruits does resemble a strange, albeit juicy ear of corn sitting on the forest floor. The bear part of the name likely has to do with the fact that bear readily consume bearcorn fruits, stem and all. Working in the southern Appalachian Mountains, I can’t tell you how many times I came across bear scat absolutely loaded with bearcorn fruits and seeds. It’s not just bear either, deer are also very interested in bearcorn fruits.
Lucky for bearcorn, its seeds pass through the guts of these animals unharmed. Hopefully, with a bit of luck, at least one of these animals will make a deposit in an oak-rich region of the forest. With even more luck, some of those seeds might even find themselves nestled in near an oak root to begin the process anew.







![(A) Young yellow-orange pseudoflower. (B) Mature pseudoflower. (C) Longitudinal section of an infected X. surinamensis inflorescence. (D) Healthy yellow flower of X. surinamensis shown for comparison. [SOURCE]](https://images.squarespace-cdn.com/content/v1/544591e6e4b0135285aeb5b6/1612389646771-T82PTY66AT9ULQO2VTPW/1-s2.0-S1087184520301572-gr1.jpg)









![Photo by Edwino S. Fernando [source]](https://images.squarespace-cdn.com/content/v1/544591e6e4b0135285aeb5b6/1550353497346-FB5R33TTIU4UMX2RE0X7/Rafflesia_consueloae_open_flower.jpg)







![Stems climbing on fallen dead wood (a) or on standing living trees (b). A thick and densely branched root clump (c) and thin and elongate roots (d) [Source]](https://images.squarespace-cdn.com/content/v1/544591e6e4b0135285aeb5b6/1518471929195-C99KP24U24J238YMX055/myco+roots.JPG)






![[SOURCE]](https://images.squarespace-cdn.com/content/v1/544591e6e4b0135285aeb5b6/1510771591218-E7STZ1TS2LEW7CZUCQFX/nph14859-fig-0001.png)













![Photo by Suetsugu Kenji [SOURCE]](https://images.squarespace-cdn.com/content/v1/544591e6e4b0135285aeb5b6/1477880480914-NI1RT36AQBR67J716TOY/image-asset.jpeg)