Photo by Eric in SF licensed under CC BY-SA 3.0
One thing I love about orchids is that they are so diverse. One could spend their entire life studying these plants and never run out of surprises. Every time I sit down with an orchid topic in mind, I end up going down a rabbit hole of immeasurable depth. I love this because I always end up learning new and interesting facts. For instance, I only recently learned that there is a genus of orchids that has been given the unbelievably complex name of Aa.
No, that is not an abbreviation. The genus was literally named Aa. As far as I have been able to tell, it is pronounced “ah” rather than “ay,” but if any linguists are reading this and beg to differ, please chime in! Regardless, I was floored by this silly exercise in plant naming and had to learn more. I had never heard of this genus before and figured that it was so obscure that it probably contained, at most, only a small handful of species. This assumption was wrong.
Aa maderoi. Photo by Dr. Alexey Yakovlev licensed under CC BY-SA 2.0
Though by no means massive, the genus Aa contains at least 25 recognized species. A quick search of the literature even turned up a few relatively recent papers describing new species. Apparently we have a ways to go in understanding their diversity. Nonetheless, this is an interesting and pretty genus of orchids.
From what I gather, Aa are most often found growing at high elevations in the Andes, though at least one species is native to mountainous areas of Costa Rica. They are terrestrial orchids that prefer cooler temperatures and fairly moist soil. Some species are said to only be found in close proximity to mountain streams. Some of the defining features of the genus are a tall inflorescence jam packed with tiny inconspicuous, greenish-white flowers. The flowers are surrounded by semi-transparent sheaths that are surprisingly showy. All in all, they kind of remind me of a mix between Spiranthes and Goodyera.
Close up of an inflorescence of Aa maderoi showing the small, white flowers and large, semi-transparent sheaths. Photo by Dr. Alexey Yakovlev licensed under CC BY-SA 2.0
But what about the name? Why in the world was this genus given such a strange and abrupt moniker? The answer seems to be the silliest option I could think of: to be first. This genus was originally described in 1845 by German botanist Heinrich Gustav Reichenbach who recognized two species within the genus Altensteinia to be distinct enough to warrant their own genus.
According to most sources I could find, he coined this new genus Aa so that it would appear first on all taxonomic lists. There is at least one other report that the name was given in honor of a man by the name of Pieter van der Aa, but apparently this is “highly” disputed. However, all of this should be taken with a grain of salt. Though I can find plenty of literature describing various species within the genus, I could turn up no actual literature on the naming of the genus itself. All I could find is what has been repeated (almost verbatim) from Wikipedia.
So, there you have it. Not only does the genus Aa exist, it is still top of the list of all plant genera. If that truly was the goal Heinrich Gustav Reichenbach was aiming for, he certainly has succeeded!
Photos via Wikimedia Commons
Further Reading: [1]



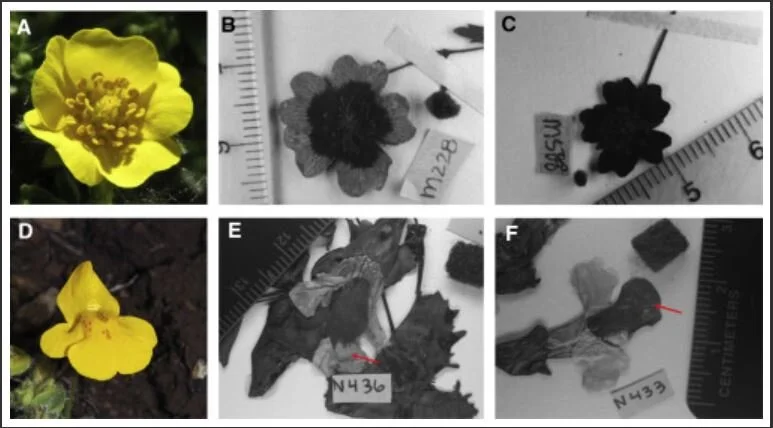
![(A) Young yellow-orange pseudoflower. (B) Mature pseudoflower. (C) Longitudinal section of an infected X. surinamensis inflorescence. (D) Healthy yellow flower of X. surinamensis shown for comparison. [SOURCE]](https://images.squarespace-cdn.com/content/v1/544591e6e4b0135285aeb5b6/1612389646771-T82PTY66AT9ULQO2VTPW/1-s2.0-S1087184520301572-gr1.jpg)

















![Current range of dwarf sumac (Rhus michauxii). Green indicates native presence in state, Yellow indicates present in county but rare, and Orange indicates historical occurrence that has since been extirpated. [SOURCE]](https://images.squarespace-cdn.com/content/v1/544591e6e4b0135285aeb5b6/1609184191069-557J58DPH2J8L74Y1O06/Rhus+michauxii.png)




![An African crested rat displaying its crest of toxic hairs and aposematic color pattern. [SOURCE]](https://images.squarespace-cdn.com/content/v1/544591e6e4b0135285aeb5b6/1606834253309-5IB7MB09H0VN2YDT1DUF/rat1.JPG)

![[Source]](https://images.squarespace-cdn.com/content/v1/544591e6e4b0135285aeb5b6/1614349162208-KK6RR3PXF0XX704I2KX2/1-s2.0-S0960982220316559-fx1.jpg)






![Even “large” basurera are not that big. [SOURCE]](https://images.squarespace-cdn.com/content/v1/544591e6e4b0135285aeb5b6/1603918941407-K909G5BOUFB85D1X6B28/ppp310161-fig-0001-m.jpg)
![Adventitious roots emerging from the stem of a basurera. [SOURCE]](https://images.squarespace-cdn.com/content/v1/544591e6e4b0135285aeb5b6/1603918982958-Z63QHN9PYJNN2FZ69KQ5/ppp310161-fig-0001-m.jpg)









![(A–C) Female Forcipomyia biting midge. Arrows indicating pollen grains. [SOURCE]](https://images.squarespace-cdn.com/content/v1/544591e6e4b0135285aeb5b6/1602516996716-ICQ7PIZDYU1BOSTTEWJ3/tacca+midge.JPG)


![The structure model of stinging tree venom (left) and the stinging trichomes of D. excelsa (right). [SOURCE]](https://images.squarespace-cdn.com/content/v1/544591e6e4b0135285aeb5b6/1601307201196-3VFCBKWMHMVZ0ZFI9P6D/sting2.JPG)
![The petioles of D. excelsa are covered in stinging hairs (top). Scanning electron micrograph of trichome structure on the leaf of D. moroides (bottom). [SOURCE]](https://images.squarespace-cdn.com/content/v1/544591e6e4b0135285aeb5b6/1601307290389-18K0E1TO357ZSG9DQLC2/sting1.JPG)