Photo by Jörg Hempel licensed under CC BY-SA 2.0
A subset of plants have evolved the ability to produce heat, a fact that may come as a surprise to many reading this. The undisputed champions of botanical thermogenesis are the aroids (Araceae). Exactly why they do so is still the subject of scientific debate but the means by which heat is produced is absolutely fascinating.
The heat producing organ of an aroid is called the spadix. Technically speaking, a spadix is a spike of minute flowers closely arranged around a fleshy axis. All aroid inflorescences have one and they come in a wide variety of shapes, colors, and textures. To produce heat, the spadix is hooked up to a massive underground energy reserve largely in the form of carbohydrates or sugars. The process of turning these sugars into heat is rather complex and surprisingly animal-like.
Cross section of a typical aroid inflorescence with half of the protective spathe removed. The spadix is situated in the middle with a rings of protective hairs (top), male flowers (middle), and female flowers (bottom). Photo by Kristian Peters -- Fabelfroh licensed under CC BY-SA 3.0
It all starts with a compound we are rather familiar with - salicylic acid - as it is the main ingredient in Aspirin. In aroids, however, salicylic acid acts as a hormone whose job it is to initiate both the heating process as well as the production of floral scents. It signals the mitochondria packed inside a ring of sterile flowers located at the base of the spadix to change their metabolic pathway.
In lieu of their normal metabolic pathway, which ends in the production of ATP, the mitochondria switch over to a pathway called the "Alternative Oxidase Metabolic Pathway." When this happens, the mitochondria start burning sugars using oxygen as a fuel source. This form of respiration produces heat.
Thermal imaging of the inflorescence of Arum maculatum.
As you can imagine, this can be a costly process for plants to undergo. A lot of energy is consumed as the inflorescence heats up. Nonetheless, some aroids can maintain this costly level of respiration intermittently for weeks on end. Take the charismatic skunk cabbage (Symplocarpus foetidus) for example. Its spadix can reach temperatures of upwards of 45 °F (7 °C) on and and off for as long as two weeks. Even more incredible, the plant is able to do this despite freezing ambient temperatures, literally melting its way through layers of snow.
For some aroids, however, carbohydrates just don't cut it. Species like the Brazilian Philodendron bipinnatifidum produce a staggering amount of floral heat and to do so requires a different fuel source - fat. Fats are not a common component of plant metabolisms. Plants simply have less energy requirements than most animals. Still, this wonderful aroid has converged on a fat-burning metabolic pathway that puts many animals to shame.
The inflorescence of Philodendron bipinnatifidum can reach temps as high as 115 °F (46 °C). Photo by Tekwani licensed under CC BY-SA 3.0
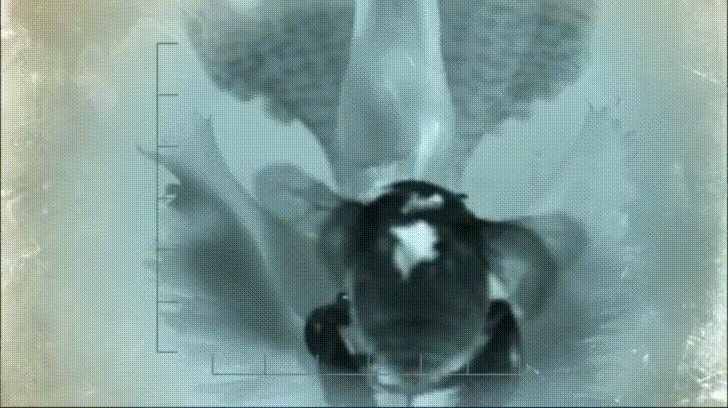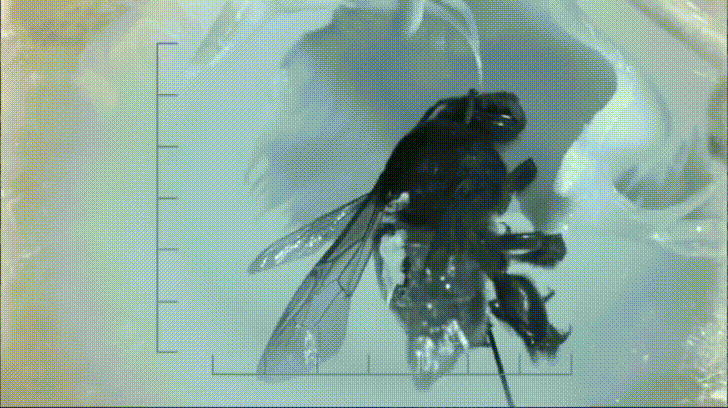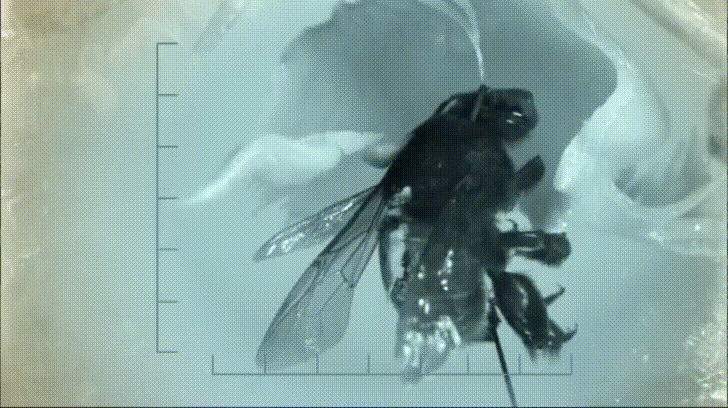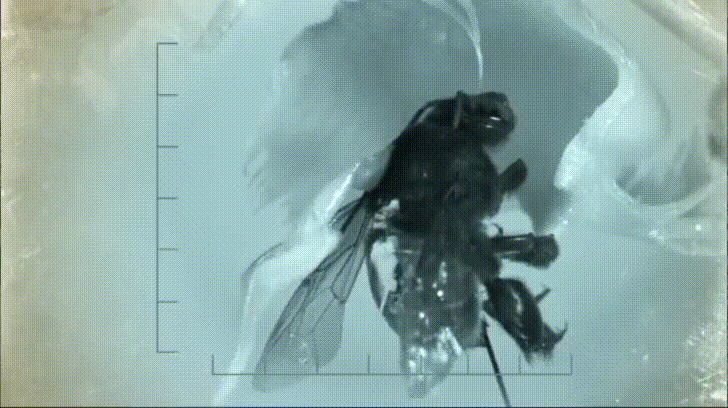
P. bipinnatifidum stores lots of fat in sterile male flowers that are situated between the fertile male and female flowers near the base of the spadix. As soon as the protective spathe opens, the spadix bursts into metabolic action. As the sun starts to set and P. bipinnatifidum's scarab beetle pollinators begin to wake up, heat production starts to hit a crescendo. For about 20 to 40 minutes, the inflorescence of P. bipinnatifidum reaches temperatures as high as 95 °F (35 °C) with one record breaker maxing out at 115 °F (46 °C)! Amazingly, this process is repeated again the following night.
It goes without saying that burning fat at a rate fast enough to reach such temperatures requires a lot of oxygen. Amazingly, for the two nights it is in bloom, the P. bipinnatifidum inflorescence consumes oxygen at a rate comparable to that of a flying hummingbird, which are some of the most metabolically active animals on Earth.
The world's largest inflorescence belongs to the titan arum (Amorphophallus titanum) and it too produces heat. Photo by Fbianh licensed under CC0 1.0
Again, why these plants go through the effort of heating their reproductive structures is still a bit of a mystery. For most, heat likely plays a role in helping to volatilize floral scents. Anyone that has spent time around blooming aroids knows that this plant family produces a wide range of odors from sweet and spicy to downright offensive. By warming these compounds, the plant may be helping to lure in pollinators from a greater distance away. It is also thought that the heat may be an attractant in and of itself. This is especially true for temperate species like the aforementioned skunk cabbage, which frequently bloom during colder months of the year. Likely both play a role to one degree or another throughout the aroid family.
What we can say is that the process of plant thermogenesis is absolutely fascinating and well worth deeper investigation. We still have much to learn about this charismatic group of plants.






























![Stems climbing on fallen dead wood (a) or on standing living trees (b). A thick and densely branched root clump (c) and thin and elongate roots (d) [Source]](https://images.squarespace-cdn.com/content/v1/544591e6e4b0135285aeb5b6/1518471929195-C99KP24U24J238YMX055/myco+roots.JPG)



![Mentzelia phylogeny showing reduction in seed wings. [source]](https://images.squarespace-cdn.com/content/v1/544591e6e4b0135285aeb5b6/1517881654366-84BORS8KUYHRY6IUK9DQ/ajb21811-fig-0002.png)